

2D visualization and quantitative analysis of the humeral diaphysis cortical thickness
Received date: 2020-02-25
Revised date: 2020-04-09
Online published: 2020-09-11
Morphometric mapping is a 2D visualization method that displays 3D morphometric information, which can effectively reflect the distribution characteristics of cortical thickness. Despite there exist differences in the distribution pattern of cortical thickness between modern humans and Pleistocene archaic humans and great apes, more explorations are needed to test whether there are variations within Holocene modern humans. In present study, 34 right humeral specimens (23 males and 11 females) from 6 Holocene central and north China agricultural populations were selected to analyze the distribution characteristics of the humeral diaphysis cortical thickness by morphometric mapping comprehensively. This work compared the differences between the analysis results obtained after standardizing the cortical thickness by maximum thickness and biomechanical length, and verified the applicability of principal component analysis on morphometric mapping. The results reveal that within the Holocene central and north China agricultural populations, there exists some variances between males and females, but males of different populations show no obvious distinction. Though this work to some extent reveals the variations in the distribution of humeral diaphysis cortical thickness of Holocene modern humans by analyzing the agricultural populations from Holocene central and northern China, it’s still necessary to carry out works on new specimens which contain diverse populations, large sample size and strict variable control by method used in the present study, so as to verify or expand the conclusions obtained here in the future.
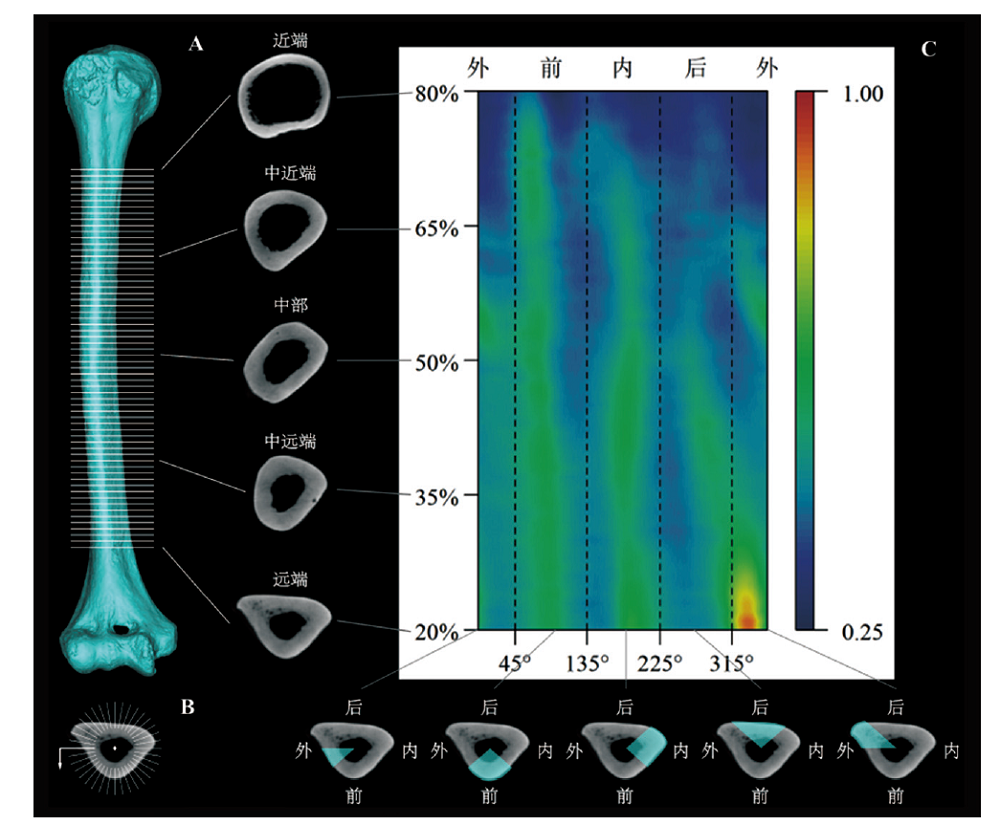
Yuhao ΖΗΑΟ , Mi ΖHOU , Pianpian WEI , Song XING . 2D visualization and quantitative analysis of the humeral diaphysis cortical thickness[J]. Acta Anthropologica Sinica, 2020 , 39(04) : 632 -647 . DOI: 10.16359/j.cnki.cn11-1963/q.2020.0021
| [1] | Morimoto N, Zollikofer CPE, Ponce de León MS. Shared human-chimpanzee pattern of perinatal femoral shaft morphology and its implications for the evolution of hominin locomotor adaptations[J]. PLOS ONE, 2012,7(7):e41980 |
| [2] | Carlson KJ, Marchi D. Reconstructing mobility[M]. New York: Springer, 2014 |
| [3] | Carlson KJ, Sumner DR, Morbeck ME, et al. Role of nonbehavioral factors in adjusting long bone diaphyseal structure in free-ranging Pan troglodytes[J]. Int J Primatol, 2008,29(6):1401-1420 |
| [4] | 吴汝康, 贾兰坡. 周口店新发现的中国猿人化石[J]. 古生物学报, 1954, 2(3): 267-288+348-354 |
| [5] | 张旭, 李婧, 朱泓. 内蒙古和林格尔县大堡山墓地古代居民的肢骨研究[J]. 人类学学报, 2015,34(2):216-224 |
| [6] | 陈德珍, 吴新智. 河南长葛石固早期新石器时代人骨的研究(续)[J]. 人类学学报, 1985,4(4):314-323 |
| [7] | Trinkaus E, Ruff CB. Femoral and tibial diaphyseal cross-sectional geometry in Pleistocene Homo[J]. PaleoAnthropology, 2012: 13-62 |
| [8] | Ruff CB, Hayes WC. Cross-sectional geometry of Pecos Pueblo femora and tibiae—A biomechanical investigation: I. Method and general patterns of variation[J]. Am J Phys Anthropol, 1983,60(3):359-381 |
| [9] | Ruff CB, Hayes WC. Cross-sectional geometry of Pecos Pueblo femora and tibiae—A biomechanical investigation: II. Sex, age, and side differences[J]. Am J Phys Anthropol, 1983,60(3):383-400 |
| [10] | Sparacello VS, Villotte S, Shackelford LL, et al. Patterns of humeral asymmetry among Late Pleistocene humans[J]. C R Palevol, 2017,16(5):680-689 |
| [11] | Ruff CB. Biomechanical analyses of archaeological human skeletons[M]. Katzenberg M A, Grauer A L. Biological anthropology of the human skeleton. Hoboken: John Wiley & Sons, Inc, 2019, 189-224 |
| [12] | Amtmann E, Schmitt HP. über die Verteilung der Corticalisdichte im menschlichen Femurschaft und ihre Bedeutung für die Bestimmung der Knochenfestigkeit[J]. Zeitschrift für Anatomie und Entwicklungsgeschichte, 1968,127(1):25-41 |
| [13] | Amtmann E. Mechanical stress, functional adaptation and the variation structure of the human femur diaphysis[M]. Berlin: Springer-Verlag, 1971 |
| [14] | Zollikofer CPE, Ponce de León MS. Computer-assisted morphometry of hominoid fossils: the role of morphometric maps[M]. Phylogeny of the Neogene hominoid primates of Eurasia. Cambridge: Cambridge University Press, 2001, 50-59 |
| [15] | Bondioli L, Bayle P, Dean C, et al. Technical note: Morphometric maps of long bone shafts and dental roots for imaging topographic thickness variation[J]. Am J Phys Anthropol, 2010,142(2):328-334 |
| [16] | Puymerail L, Ruff CB, Bondioli L, et al. Structural analysis of the Kresna 11 Homo erectus femoral shaft (Sangiran, Java)[J]. J Hum Evol, 2012,63(5):741-749 |
| [17] | Puymerail L, Volpato V, Debénath A, et al. A Neanderthal partial femoral diaphysis from the “grotte de la Tour”, La Chaise-de-Vouthon (Charente, France): Outer morphology and endostructural organization[J]. C R Palevol, 2012,11(8):581-593 |
| [18] | Zanolli C, Bondioli L, Coppa A, et al. The late Early Pleistocene human dental remains from Uadi Aalad and Mulhuli-Amo (Buia), Eritrean Danakil: Macromorphology and microstructure[J]. J Hum Evol, 2014,74:96-113 |
| [19] | Jashashvili T, Dowdeswell MR, Lebrun R, et al. Cortical Structure of Hallucal Metatarsals and Locomotor Adaptations in Hominoids[J]. PLOS ONE, 2015,10(1):e0117905 |
| [20] | Morimoto N, Ponce de León MS, Zollikofer CPE. Exploring femoral diaphyseal shape variation in wild and captive chimpanzees by means of morphometric mapping: a test of Wolff’s law[J]. The Anatomical Record, 2011,294(4):589-609 |
| [21] | Puymerail L. The functionally-related signatures characterizing the endostructural organisation of the femoral shaft in modern humans and chimpanzee[J]. C R Palevol, 2013,12(4):223-231 |
| [22] | Wei PP, Wallace IJ, Jashashvili T, et al. Structural analysis of the femoral diaphyses of an early modern human from Tianyuan Cave, China[J]. Quat Int, 2017,434:48-56 |
| [23] | Ruff C. Growth tracking of femoral and humeral strength from infancy through late adolescence[J]. Acta Paediatr, 2005,94(8):1030-1037 |
| [24] | Sumner DR, Andriacchi TP. Adaptation to differential loading: Comparison of growth-related changes in cross-sectional properties of the human femur and humerus[J]. Bone, 1996,19(2):121-126 |
| [25] | Morimoto N, Nakatsukasa M, Ponce de León MS, et al. Femoral ontogeny in humans and great apes and its implications for their last common ancestor[J]. Sci Rep, 2018,8(1):1930 |
| [26] | Dupej J, Lacoste Jeanson A, Pelikán J, et al. Semiautomatic extraction of cortical thickness and diaphyseal curvature from CT scans[J]. Am J Phys Anthropol, 2017,164(4):868-876 |
| [27] | 邵象清. 人体测量手册[M]. 上海: 上海辞书出版社, 1985 |
| [28] | 吴汝康, 吴新智, 张振标. 人体测量方法[M]. 北京: 科学出版社, 1984 |
| [29] | Ruff CB. Long bone articular and diaphyseal structure in old world monkeys and apes. I: Locomotor effects[J]. Am J Phys Anthropol, 2002,119(4):305-342 |
| [30] | R Development Core Team. R: A language and environment for statistical computing[M]. R Foundation for Statistical Computing. Vienna, Austria. 2019 |
| [31] | Bishop CM. Pattern recognition and machine learning[M]. New York: Springer-Verlag, 2006 |
| [32] | 何晓群. 多元统计分析[M]. 北京: 中国人民大学出版社, 2019 |
| [33] | 费宇, 郭民之, 陈贻娟. 多元统计分析——基于R[M]. 北京: 中国人民大学出版社, 2014 |
| [34] | Klovan JE. R-and Q-Mode Factor Analysis[M]. McCammon R B. Concepts in Geostatistics. Berlin: Springer Berlin, 1975, 21-69 |
| [35] | Mar?o PH, Scarminio IS. Q-mode curve resolution of UV-vis spectra for structural transformation studies of anthocyanins in acidic solutions[J]. Anal Chim Acta, 2007,583(1):138-146 |
| [36] | Paatero P, Tapper U. Analysis of different modes of factor analysis as least squares fit problems[J]. Chemometrics Intell Lab Syst, 1993,18(2):183-194 |
| [37] | Steyn M, ??can MY. Osteometric variation in the humerus: sexual dimorphism in South Africans[J]. Forensic SciInt, 1999,106(2):77-85 |
| [38] | Sládek V, Ruff CB, Berner M, et al. The impact of subsistence changes on humeral bilateral asymmetry in Terminal Pleistocene and Holocene Europe[J]. J Hum Evol, 2016,92:37-49 |
| [39] | Lovejoy CO, McCollum MA, Reno PL, et al. Developmental biology and human evolution[J]. Annu Rev Anthropol, 2003,32(1):85-109 |
| [40] | Chiu CH, Hamrick MW. Evolution and development of the primate limb skeleton[J]. Evol Anthropol, 2002,11(3):94-107 |
| [41] | Pearson OM, Lieberman DE. The aging of Wolff’s “law”: Ontogeny and responses to mechanical loading in cortical bone[J]. Am J Phys Anthropol, 2004,125(S39):63-99 |
| [42] | Ruff C, Holt B, Trinkaus E. Who’s afraid of the big bad wolff? “Wolff is law” and bone functional adaptation[J]. Am J Phys Anthropol, 2006,129(4):484-498 |
| [43] | Hsieh YF, Robling AG, Ambrosius WT, et al. Mechanical Loading of Diaphyseal Bone In Vivo: The Strain Threshold for an Osteogenic Response Varies with Location[J]. J Bone Miner Res, 2001,16(12):2291-2297 |
| [44] | Burr DB, Robling AG, Turner C H. Effects of biomechanical stress on bones in animals[J]. Bone, 2002,30(5):781-786 |
| [45] | Niinim?ki S, S?derling S, Junno JA, et al. Cortical bone thickness can adapt locally to muscular loading while changing with age[J]. Homo, 2013,64(6):474-490 |
/
| 〈 |
|
〉 |